Cyanokit
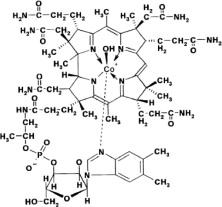
What is Cyanokit (Hydroxocobalamin)?
Related Clinical Trials
Summary: The clinical trial is a phase 1, single-arm trial that will evaluate the safety of the investigational treatment on metastatic cancer in patients who have a deleterious or suspected deleterious BRCA1, BRCA2, or PALB2 genetic alteration. The investigational treatment will involve 2 cycles of a combination of intravenous melphalan, BCNU, low-dose I.V. ethanol, vitamin B12b, and vitamin C in associat...
Summary: This study is a clinical trial being done to investigate the efficacy of drug BRS201 (hydroxocobalamin) as a treatment in patients with primary sclerosing cholangitis. Participation in this study will take 8 weeks long and the study is structured as a cross-over study in which participants will take the study drug for 4 weeks and a placebo drug for 4 weeks in a randomized order in the form of an o...
Summary: The goal of this study is to use pain-specific urine biomarkers to evaluate how daily nutritional supplementation with biomarker guided formulas effect, quality of life and urinary biomarker scores in chronic pain patients. Assessing the effect of biomarker guided supplementation on pain specific biomarkers through changes in urinary biomarker scores may solidify the necessity for identifying defi...
Related Latest Advances
Brand Information
- One 250 mL glass vial, containing lyophilized hydroxocobalamin for injection, 5 g
- One sterile transfer spike
- One sterile intravenous infusion set
- One quick use reference guide
- One package insert